Abstract
Background: Hypomethylating agents (HMAs) (5-azacytidine [Aza] or decitabine [Dec]) combined with venetoclax (Ven) is standard treatment for acute myeloid leukemia (AML) patients unfit for induction chemotherapy. Though less toxic than standard induction, pulse-cycled HMA with daily Ven (standard HMA/Ven) frequently cause myelosuppression which undermine treatment goals and sustainability. We describe real-world experience using an alternative HMA/Ven regimen (LDDec/Ven) modified to address this limitation.
Methods: HMA Dose: HMA-targeting of DNA methyltransferase is saturated at low doses/concentrations, but higher concentrations cause cytotoxicity to normal myeloid cells; we hence identified and administered minimum Dec doses needed to deplete DNMT1 without cytotoxicity in non-human primates and in clinical trials (0.1-0.2 mg/kg)(LDDec) (Saunthararajah et al. J Clin Invest 2015; Awada et al. Br J Haematol 2020). HMA Schedule: Since DNMT1-targeting is exposure-time (S-phase) dependent, and serial daily administrations rapidly educate for adaptive metabolic responses and resistance, we scheduled frequent-distributed 1-2X/week instead of pulse-cycled administration (Gu et al, Leukemia 2021). Ven Schedule: Although Ven has limited single-agent activity to treat AML, it leverages BCL2-dependence created in malignant myeloid cells by prior HMA or cytarabine exposure (Jin et al. Clin Ca Res. 2020). Thus, to improve safety/feasibility but retain benefits of combination therapy, Ven was scheduled 1X/week to overlap with Dec exposures 1-2X/week.
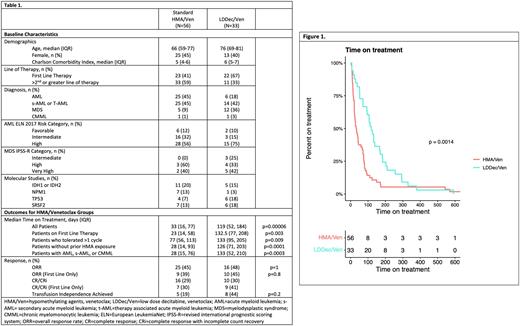
We performed a retrospective review of patients >18 years of age with MDS, AML, and CMML treated with standard HMA/Ven or LDDec/Ven at Montefiore Medical Center between 11/2018 and 08/2021. Primary outcomes included time-on-treatment defined as start-of-therapy to last-dose of Ven or end of final cycle. Treatment delays >7 days were considered treatment interruptions. Secondary outcomes included best overall response (ORR) (composite CR, CRi, MLFS, and PR), overall survival (OS), and transfusion-independence. Continuous variables including time on treatment were analyzed by Kruskal-Wallis test, with nominal variables analyzed by Fischer's Exact Test (Table 1). The percentage of patients who remained on treatment was also estimated using the Kaplan-Meier method (Figure 1). A Cox proportional hazards model was used to estimate hazard ratio of OS.
Results: We found 89 patients received HMA/Ven: Aza/Ven (n=30), Dec/Ven (n=26), or LDDec/Ven (n=33). LDDec/Ven treated patients were significantly older vs the other cohorts (median age 76 vs 66 years). LDDec/Ven cohort had 36% of patients with MDS vs 9% in other cohorts, and LDDec/Ven was first-line therapy in 67% vs 41% in other cohorts. There was no significant difference in the median ECOG PS (1) or Charlson Comorbidity Index scores (5 and 6 for LDDec/Ven and HMA/Ven, respectively). 61% of AML patients had poor risk cytogenetics, and 82% of MDS patients were high or very high risk. More patients in the LDDec/Ven cohort were treated as first-line therapy than standard HMA/Ven (67% vs 41%).
Median time on treatment with LDDec/Ven was 119 vs 33 days for other cohorts (p=0.00006) with 26 patients (43%) in the standard HMA/Ven cohort not completing the first cycle of therapy. When restricted to patients who received at least two cycles of therapy the difference was 133 vs 76 days (Table 1). There was no difference in OS (HR 1.18, p=0.53). In transfusion-dependent patients, transfusion-independence was achieved in 44% of LDDec/Ven vs 19% of other cohorts (p=0.17). In patients with AML, ORR was 45% of LDDec/Ven vs 48% of other cohorts (p=0.82); 33% of patients were not evaluable for ORR due to missing pathological data).
Discussion: In this single-institution retrospective study, patients treated with LDDec/Ven 1-2x/week had significantly longer time-on-treatment than patients treated with standard pulse-cycled HMA with daily-Ven, and similar overall survivals despite their significantly older age. Therefore, it may be possible to simultaneously preserve or increase HMA/Ven efficacy and decrease myelosuppression/toxicity by using recent understanding of mechanisms to guide regimen design, although this will require a prospective study: a prospective clinical trial evaluating LDDEC/Ven in MDS and AML (NCT05184842) is actively enrolling patients.
Disclosures
Shastri:Janssen: Consultancy; Rigel Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; NACE: Honoraria; Kymera Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Sica:MorphoSys: Consultancy, Honoraria; Miragen: Consultancy, Honoraria; Kite Pharma: Research Funding; Bristol Myers Squibb Foundation: Research Funding; AstraZeneca: Honoraria, Research Funding; Curios: Honoraria; PER Physician's Education Review.: Consultancy, Honoraria. Feldman:Ichnos Sciences: Current Employment, Current holder of stock options in a privately-held company. Verma:Eli Lilly: Research Funding; Stelexis Therapeutics: Current equity holder in private company, Honoraria; Bakx Therapeutics: Consultancy, Current equity holder in private company; Ionctura: Research Funding; Novartis: Consultancy; Jannsen: Consultancy, Research Funding; Prelude: Research Funding; Curis: Consultancy, Research Funding; Medpacto: Research Funding; BMS: Research Funding; Throws Exception: Current holder of stock options in a privately-held company. Saunthararajah:EpiDestiny: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: intellectual property with royalty rights ; Novo Nordisk: Consultancy.
OffLabel Disclosure:
Decitabine and venetoclax on an alternative dosing regimen for AML
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal